VERTELLUS Drug Eluting Stent Coating Drug Delivery Stent Coating

Introduction to PC technology
PC, short for phosphorylcholine, is the chemical name for the polar head group present in a variety of phospholipids, especially those that make up the bilayer of cell membranes.
PC Technology is a proprietary technology platform for phosphorylcholine-containing methacrylic polymers. These biomimetic materials are built on the body's own chemistry, but they are entirely synthetic, allowing precise control over purity, molecular structure, and avoiding the risks associated with animal-derived ingredients. They can provide a simple and effective solution to the bioscientific challenges faced by medical devices and can be tailored to specific application needs.
Product List
Ventrous Biomaterials offers a wide range of polymer coatings and monoliths to take advantage of the various properties and benefits that PCs can provide:
|
polymer |
Briefly |
application |
|
PC1036 |
Standard cross-linked PC coating, high durability drug delivery matrix |
BiodivYsio® Dexamet® TriMaxx™, Endeavor® Stent |
|
PC1059 |
Standard non-crosslinked PC coatings stabilized by hydrophobic surface interactions |
Guide wire, CPB system |
|
PC2118 |
Cross-linked PC coating, high content of PC forms a self-wetting surface |
blood filtration and processing |
|
PC1062 |
Cross-linked PC coating, with low content of positive charge, can bind heparin to form an antithrombotic coating |
blood filtration and processing |
|
PC2028 |
Cross-linked PC coating, high content of positive charge, can be used as a matrix for high molecular weight drug delivery |
BiodivYsio Matrix Hi Stand |
|
PC1071 |
In-mold PC coatings with vinyl functional groups for curable materials such as silicones |
functional product |
|
PC1015 |
Cross-linked monolithic PC hydrogel material suitable for mold forming |
Proclear® Contact Lenses |
|
PC2083 |
Flexible monolithic PC hydrogel with high reflectivity index for mold forming |
functional product |
·Integral PC biomaterials and coatings can provide solutions for stents, guidewire flow path elements, oxygenators, dialysis elements, intraocular lens drains, shunts, filters, dental materials, catheters, bone fixation wires, and screws.
Recognized and used by a number of medical device companies, such as Abbott, Medtronic, Cooper Optics, and Italian Sorin Group, the three FDA-allowed label claims demonstrate the clinical benefits of PC technology.
· This technology is also supported by a wide range of patents and FDA Master files.
PC Technology™ improves clinical performance
There are numerous reports detailing the results of clinical and in vitro studies, some of which are as follows:
a. Hemovolemia
"In short-term clinical applications, PC coatings are beneficial in preventing guidewire tip thrombosis..."
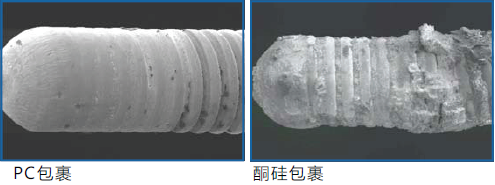
b. Resist bacterial adhesion and biofilm formation
"This coating has been shown to resist the formation of Staphylococcus aureus and Pseudomonas aeruginosa biofilms..."
Application of PC Technology™ in the Medical Field
In a variety of applications, PC materials have been shown to improve the biocompatibility and performance of medical devices and materials by reducing:
o Protein deposition/activation o Biofilm deposition
o Blood activation/thrombosis o Inflammatory response
o Bacterial Adhesion o Fibrocyst Formation
Additionally, PC biomaterials have been shown to be good substrates for the controlled delivery of multiple active ingredients.
PC biomaterials have been successfully used to encapsulate the following substrates:
o Metals, including stainless steel, nitinol, titanium, gold, platinum
o Plastics, including polyolefin, polyvinyl chloride, PMMA, PET, polyurethane, polycarbonate, polyamide, polyimide, polystyrene, teflon
o Rubber, including silicone, latex, PIB
o Glass and Ceramics
o Tooth enamel and other tissues
Integral PC biomaterials and coatings can be supplied for stents, guide wires, blood flow path elements, oxygenators, dialysis elements, intraocular lenses, drain tubes, shunts, filters, dental materials, catheters, bone fixation wires and screws, etc. The workaround, in part, is as follows:
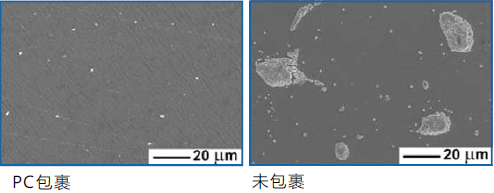
a. Reduce protein deposition

Filter with PC technology Filter without PC technology
b. Reduce blood activation

blood filter with PC technology blood filter without PC technology
c. Reduce biofilm formation

Ureter with PC technology Ureter without PC technology
Application of PC Technology™ in non-medical fields
Clearly, the non-fouling and surfactant properties of PC have potential benefits in non-medical applications.
Some examples of applicable properties, benefits, and potential applications are summarized below:
|
Application of PC Technology™ in Drug Delivery
In recent years, the development of drug-device combination products has brought about significant changes in some parts of the medical device field. Perhaps the clearest example has always been the drug-eluting stent (DES) market, which has exploded from $1.5 billion to $5 billion in two years. PC coating plays an important role in this field. Abbott's PC ethomethasone stent, Dexamet® is the second DES to market in Europe, and Medtronic's PC-coated otarolimus-eluting Endeavor® DES system, strikingly after a two-year follow-up of 1,300 patients In the test, there was no stent thrombosis at night. These application examples highlight the value of PC materials to the medical device market.
The flexible chemistry of PC polymers enables modification of various monomers, allowing tailoring of polymers for different actives and fine-tuning of drug elution properties. Breakthrough drug-device combinations that demonstrate clinical advantage through controlled drug release, Vantruth's expertise is well suited to serve the development and commercialization of combination products.
PC coating can:
o Adsorption of high molecular weight compounds
o Absorption of low molecular weight drugs
o Demonstrates precise drug content and controlled release
o Once the drug is released, it remains stable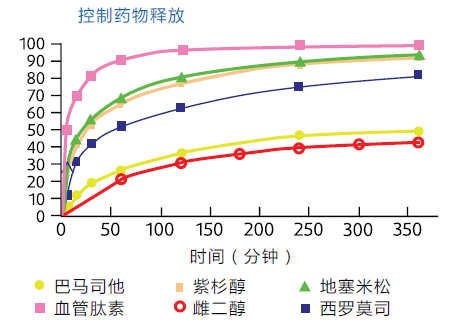
PC coatings are able to deliver:
o Anti-inflammatory drugs o Pain relievers
o Antimicrobials o Hormones
o DNA fragments used in gene therapy
Specific examples of active substances that have been studied:
o Dexamethasone o Estradiol
o Paclitaxel o Sirolimus
o Bamatestat o Angiopeptin
o Dipyridamole
Manufacturer information
The headquarters and application laboratory of Vantruth Biomaterials is located in Hampshire, UK. The division is part of Ventrus Specialty Products UK Ltd, based in the North of England, which operates under an ISO9001:2008 quality management system (certificate number FM02222). Ventrus Specialties UK Ltd has facilities for the production of multi-ton PC materials and has recently opened a new polymer chemistry research and development laboratory to support operations in the Biomaterials division. Vantruth is ideally suited to assist medical supply companies in applying the benefits of PC technology to address unmet clinical needs and provide value-added, differentiated products.
- PREV:BASF Dispex® anionic dispersant based on polyacrylic acid AA4040…
- NEXT:Cashew Nut Shell Oil Diol FX-9201LP





















